Profound Medical develops customizable, incision-free therapies which combine real-time Magnetic Resonance (“MR”) imaging, thermal ultrasound and closed-loop temperature feedback control for the radiation-free ablation of diseased tissue.
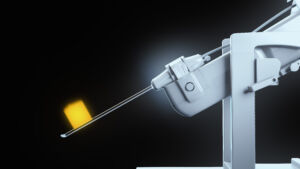
Profound is commercializing TULSA-PRO®, a technology that combines real-time MRI, robotically-driven transurethral ultrasound and closed-loop temperature feedback control. TULSA-PRO® is designed to provide customizable and predictable radiation-free ablation of a surgeon-defined prostate volume while actively protecting the urethra and rectum to help preserve the patient’s natural functional abilities.
We believe TULSA-PRO® is demonstrating to be a flexible technology in customizable prostate ablation, including intermediate stage cancer, localized radio-recurrent cancer, retention and hematuria palliation in locally advanced prostate cancer, and the transition zone in large volume benign prostatic hyperplasia (BPH). TULSA-PRO® is CE marked and received 510(k) clearance from the U.S. Food and Drug Administration in August 2019.

Profound is also commercializing Sonalleve®, an innovative therapeutic platform that is CE marked for the treatment of uterine fibroids and palliative pain treatment of bone metastases. Sonalleve® has also been approved by the China Food and Drug Administration for the non-invasive treatment of uterine fibroids.
The Company is in the early stages of exploring additional potential treatment markets for Sonalleve® where the technology has been shown to have clinical application, such as non-invasive ablation of abdominal cancers and hyperthermia for cancer therapy.
For more information visit the Profound Medical website.