The ALTA Klinik, located in Germany, offers the TULSA Procedure to men with prostate cancer seeking minimally invasive treatment. In order to assess the benefits of the TULSA Procedure in a real-world setting, the ALTA Klinik team performed a clinical service evaluation of 52 men with low (13%), medium (67%), or high-risk (19%) prostate cancer receiving the TULSA Procedure either as initial treatment for prostate cancer or for recurrent disease after other treatments failed. The results from this evaluation were published in Urologic Oncology.
Background
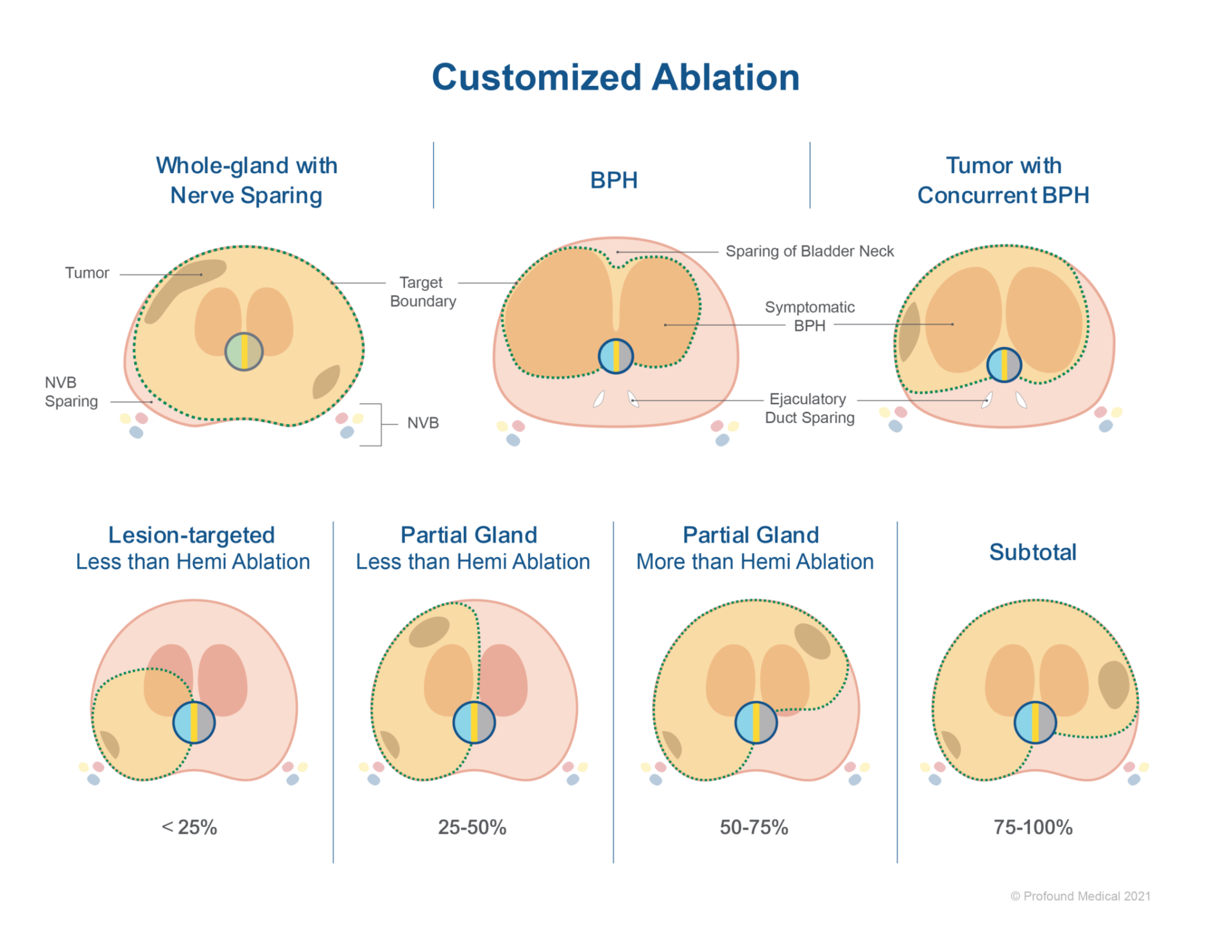
During this study, 24 men (46%) received the TULSA Procedure to target both their prostate cancer, and their benign prostate hyperplasia (BPH) during a single procedure (this is known as a combination treatment). Each patient received a customized patient-specific treatment plan, that was tailored to their specific disease and anatomy. To reduce the risk of incontinence and erectile dysfunction, a boundary line was drawn 3 mm away from the urinary sphincter and, where feasible, neurovascular bundles were spared.
Methodology
According to the authors of the study, the male participants had a prostate volume that ranged from 17-122 mL, and the volume of prostate tissue that was ablated, ranged from less than 50% to whole gland ablation. Approximately 16 months (the median follow-up time) after the TULSA Procedure, patients were assessed on the following items: treatment success for prostate cancer, relief of lower urinary tract symptoms (LUTS) for men receiving combination treatment, adverse events, urinary continence and potency preservation (ability to reach an erection or to achieve an orgasm).
Results
After a single TULSA Procedure, early treatment success for prostate cancer was achieved in 38 men (73%). Of the 9 men who chose to repeat TULSA Procedure, 8 achieved early treatment success at around 14 months (median follow-up time) increasing the rate of early treatment success after one or two TULSA Procedures to 88%.
- The majority of men (80%) underwent partial ablation.
- All men who were previously fully potent, and 98% of men with full or partial potency before treatment maintained their erectile function.
- Pad-free continence was preserved in 98% of men.
- After the combination treatment targeting prostate cancer and BPH, improvement in LUTS was achieved for 83% of men.
- Two complications requiring intervention (without general anesthesia) were observed, and there were no bowel-related complications.
Conclusions
The results should be interpreted in the context of the risk group for prostate cancer and the treatment plan. As the risk group increases and the volume of the prostate gland treated decreases, the greater the chance is of cancer recurring (as is with other therapies). However, decreasing the volume of the gland treated resulted in improved continence, improved potency preservation, and fewer adverse events.
Based on these results, the authors of this study found the TULSA Procedure to be safe and effective at treating low to high-risk prostate cancer in the real-world setting, and also at simultaneously relieving LUTS through customized treatment plans, both as a primary treatment or a salvage treatment after other modalities fail. Repeat TULSA Procedure is feasible if needed to achieve definitive treatment.
To learn more about the ALTA Klinik, visit: https://tulsaprocedure.com/alta-klinik/
To learn more about this study, visit: https://www.sciencedirect.com/science/article/pii/S1078143921001733?via%3Dihub
Oct 4, 2021 | TULSA Procedure
 Find a Center
Find a Center Contact Us
Contact Us